In the realm of pharmaceutical and biotechnology manufacturing, maintaining stringent environmental conditions is crucial for ensuring product quality and safety. In GMP-compliant environments, the stakes are particularly high. The dynamic nature of cleanroom environments necessitates continuous monitoring to uphold the integrity of these controlled spaces. Monitoring refers to the automated recording of parameters and the issuance of alarms and error messages. Significant differences exist in the implementation of monitoring systems, depending on the cleanroom classifications and standards. At ABN Cleanroom Technology, we offer two types of monitoring package for our pre-engineered cleanroom solutions: CleanConnect for environments according to ISO14644 and GMPConnect for environments according to GMP standards.
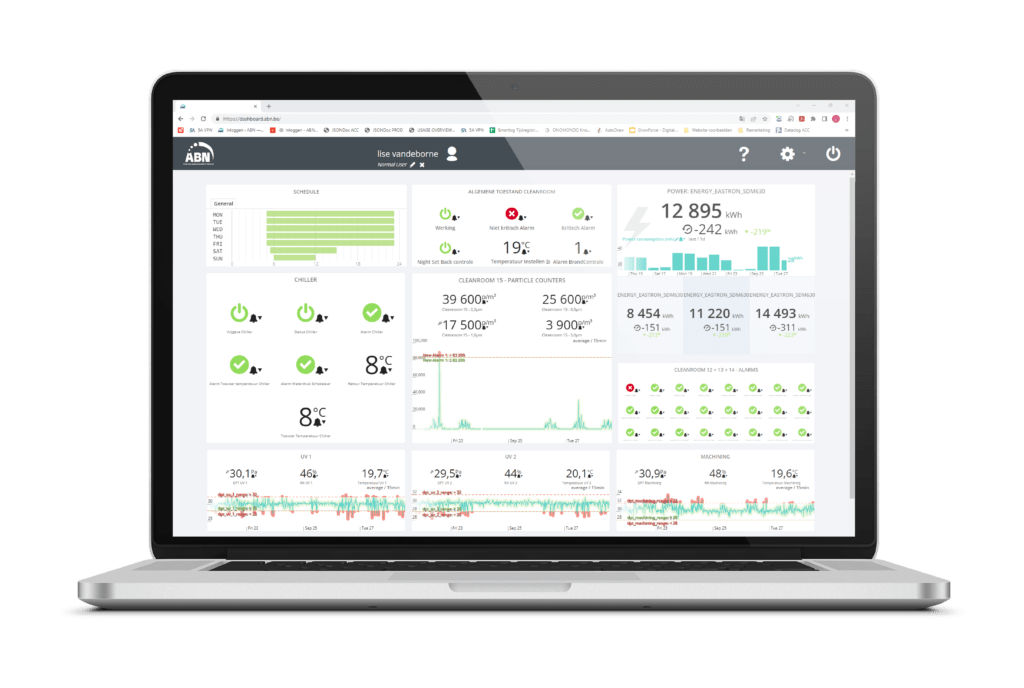
ISO monitoring involves the automated measurement and recording of data to oversee critical processes. This typically includes systematic monitoring of pressure conditions, temperature, relative humidity levels, VOCs, AMC levels, energy usage, and the performance of key technical installations such as chillers and AHUs. The CleanConnect cloud platform provides a reliable, flexible, and efficient solution for online monitoring and data reporting. With its browser-based interface, users always have access to the latest software without the need for downloads or updates. The recorded data can be archived by clients for documentation purposes.
CleanConnect offers a user-friendly and robust solution for maintaining optimal environmental conditions and ensuring compliance with ISO standards.
CleanConnect features:
GMP (Good Manufacturing Practice) monitoring differs from ISO monitoring primarily in its requirement for tamper-proof data recording. Therefore, an independent monitoring system, separate from the cleanroom regulation system, must be installed. The design of monitoring software within the GMP context is governed by the GAMP 5 (Good Automated Manufacturing Practice) guidelines. These guidelines provide a structured approach to validating automated systems, ensuring consistent and controlled operation. Additionally, an audit trail is required to monitor changes and deletions in the data, often implemented through databases.
GMPConnect provides a robust solution for maintaining data integrity and ensuring compliance with GAMP5 and GMP regulations, making it an essential tool for regulated industries.

Key features of GMPConnect monitoring system:

The interval for data recording is a frequently debated topic. Before setting these intervals, it is crucial to determine which information and proof are genuinely required. Evaluating large datasets can be labor-intensive and significantly increase the time needed for data analysis. Effective stakeholder management plays a critical role in making informed data recording decisions. ABN Cleanroom Technology has profound expertise in stakeholder management, ensuring that we understand which processes are important for the cleanroom user. By consulting with the correct stakeholders, we can provide tailored advice on data recording practices that meet the specific needs and regulatory requirements of our clients.
Our approach ensures that the data recording intervals are set optimally, balancing the need for comprehensive monitoring with the practicalities of data storage and analysis. Through strategic stakeholder engagement, ABN Cleanroom Technology facilitates informed decision-making, ensuring that all relevant parties are considered and that the data recording strategy aligns with the overall objectives of the cleanroom environment.
Legolisation means standardisation. Standardisation causes a shift in production. Work is carried out in conditioned spaces such as factory halls. our cleanrooms are manufactured partly or entirely off-site, which means huge savings on transport costs and reduction of inconvenience on-site